How Many Moles Are In 25.0 Grams Of Water
faraar
Sep 13, 2025 · 6 min read
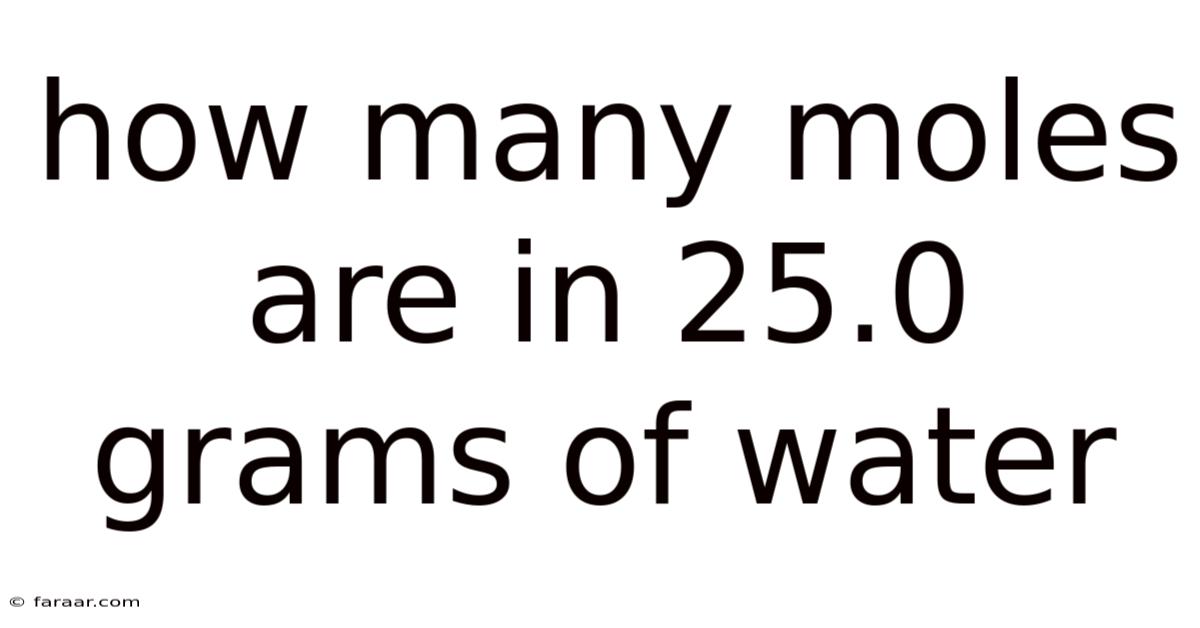
Table of Contents
How Many Moles Are in 25.0 Grams of Water? A Comprehensive Guide
Determining the number of moles in a given mass of a substance is a fundamental concept in chemistry. This article will guide you through the process of calculating the number of moles in 25.0 grams of water, explaining the underlying principles and providing a step-by-step approach. We'll delve into the relevant concepts, including molar mass, Avogadro's number, and the importance of stoichiometry in chemical calculations. This guide is designed for students and anyone interested in understanding basic chemical calculations.
Understanding Moles and Molar Mass
Before we begin our calculation, let's review some essential concepts. A mole (mol) is a fundamental unit in chemistry that represents a specific number of particles, whether atoms, molecules, ions, or other entities. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>. One mole of any substance contains Avogadro's number of particles.
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's essentially the average atomic mass of all the atoms in a molecule, considering the isotopes and their abundance. For example, the molar mass of carbon (C) is approximately 12.01 g/mol, while the molar mass of oxygen (O) is approximately 16.00 g/mol.
Calculating the Molar Mass of Water (H₂O)
Water (H₂O) is a simple molecule composed of two hydrogen atoms and one oxygen atom. To calculate its molar mass, we need the atomic masses of hydrogen and oxygen:
- Hydrogen (H): Approximately 1.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Therefore, the molar mass of water is:
(2 x 1.01 g/mol) + (1 x 16.00 g/mol) = 18.02 g/mol
Step-by-Step Calculation: Moles in 25.0 Grams of Water
Now, let's calculate the number of moles in 25.0 grams of water. We'll use the following formula:
Number of moles = (Mass of substance in grams) / (Molar mass of substance in g/mol)
-
Identify the given information: We know the mass of water is 25.0 grams.
-
Identify the molar mass: We calculated the molar mass of water as 18.02 g/mol.
-
Apply the formula:
Number of moles = (25.0 g) / (18.02 g/mol)
Number of moles ≈ 1.39 mol
Therefore, there are approximately 1.39 moles in 25.0 grams of water.
Understanding the Significance of the Result
The result, 1.39 moles, tells us that 25.0 grams of water contains approximately 1.39 times Avogadro's number of water molecules. To find the exact number of molecules, we would multiply 1.39 moles by Avogadro's number (6.022 x 10<sup>23</sup> molecules/mol):
Number of molecules ≈ 1.39 mol x 6.022 x 10<sup>23</sup> molecules/mol ≈ 8.37 x 10<sup>23</sup> molecules
This demonstrates the vast number of molecules present even in a small amount of water.
The Importance of Significant Figures
It's crucial to pay attention to significant figures in scientific calculations. In this case, the mass of water (25.0 g) has three significant figures. The molar mass of water (18.02 g/mol) also effectively has four significant figures (although the uncertainty might be higher depending on the source of atomic mass data). Therefore, the final answer should also reflect this precision, which is why we rounded the result to two decimal places (1.39 mol). Using more or fewer significant figures could lead to an inaccurate representation of the measurement.
Applications of Mole Calculations
The ability to convert between mass and moles is crucial in many areas of chemistry. It's essential for:
- Stoichiometry: Balancing chemical equations and calculating the amounts of reactants and products in chemical reactions. Knowing the number of moles allows us to determine the ratios of different substances involved in a reaction.
- Solution Chemistry: Calculating the concentration of solutions (e.g., molarity) and determining the amount of solute needed to prepare a solution of a specific concentration.
- Titrations: Using the moles of reactants to determine the unknown concentration of a solution.
- Gas Laws: Applying the ideal gas law (PV=nRT) to calculate the volume, pressure, or temperature of a gas, where 'n' represents the number of moles.
Further Exploration: Isotopic Abundance and Molar Mass
The molar mass we used (18.02 g/mol) is an average molar mass, taking into account the naturally occurring isotopes of hydrogen and oxygen. Hydrogen has two main isotopes, protium (<sup>1</sup>H) and deuterium (<sup>2</sup>H), while oxygen has several stable isotopes, including <sup>16</sup>O, <sup>17</sup>O, and <sup>18</sup>O. The relative abundance of these isotopes influences the average atomic mass and thus the molar mass of water. For highly precise calculations, it is important to consider the isotopic composition of the water sample.
FAQ: Frequently Asked Questions
-
Q: What if I had a different mass of water?
A: You would simply substitute the given mass into the formula: Number of moles = (Mass of water in grams) / (18.02 g/mol).
-
Q: Can I use this calculation for other substances?
A: Yes, this method applies to any substance. You would need to determine the molar mass of the substance in question first.
-
Q: How accurate is this calculation?
A: The accuracy depends on the precision of the mass measurement and the values used for atomic masses. Using more precise atomic mass data will improve the accuracy.
-
Q: What is the difference between a molecule and a mole?
A: A molecule is a single unit of a substance, while a mole is a large collection of molecules (approximately 6.022 x 10<sup>23</sup> molecules).
-
Q: Why is Avogadro's number so important?
A: Avogadro's number provides a bridge between the macroscopic world (grams) and the microscopic world (atoms and molecules), allowing us to relate mass to the number of particles.
Conclusion
Calculating the number of moles in a given mass of a substance is a fundamental skill in chemistry. This article provided a comprehensive guide to calculating the number of moles in 25.0 grams of water, walking through each step and emphasizing the importance of significant figures and the underlying principles of molar mass and Avogadro's number. Understanding these concepts is essential for mastering stoichiometry and many other crucial areas of chemistry. Remember to practice these calculations using different masses and substances to solidify your understanding. By understanding these fundamental concepts, you'll be well-equipped to tackle more complex chemical problems and further your exploration of the fascinating world of chemistry.
Latest Posts
Latest Posts
-
What Is The Greatest Common Factor Of 9 And 21
Sep 13, 2025
-
Spanish Classes In Orange County Ca
Sep 13, 2025
-
How To Find Equation Of A Secant Line
Sep 13, 2025
-
Sec X Cos X Sin X
Sep 13, 2025
-
How To Rewrite Without An Exponent
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about How Many Moles Are In 25.0 Grams Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.