Fecl3 Naoh Fe Oh 3 Nacl
faraar
Sep 22, 2025 · 6 min read
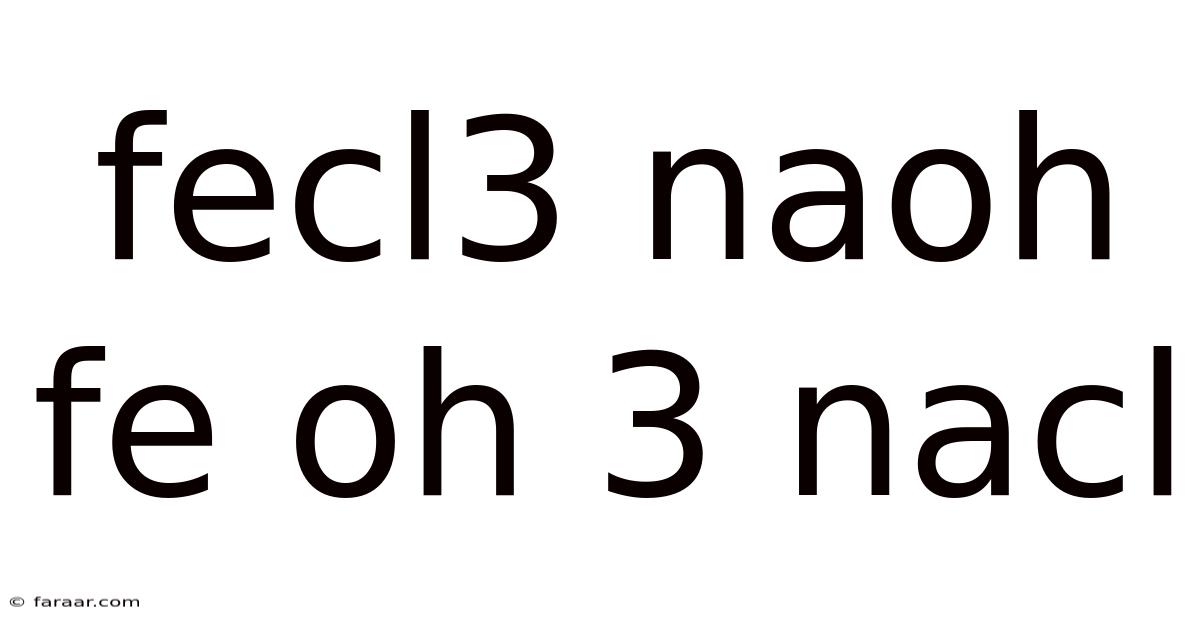
Table of Contents
The Reaction Between Ferric Chloride (FeCl3) and Sodium Hydroxide (NaOH): A Deep Dive into Precipitation Reactions
This article explores the fascinating chemical reaction between ferric chloride (FeCl₃) and sodium hydroxide (NaOH), resulting in the formation of ferric hydroxide (Fe(OH)₃) precipitate and sodium chloride (NaCl). We'll delve into the specifics of this reaction, examining its balanced equation, the underlying principles, practical applications, and potential safety considerations. Understanding this reaction is crucial for students of chemistry, as it exemplifies a fundamental type of chemical process: precipitation. This reaction also demonstrates the principles of ionic bonding, solubility, and stoichiometry.
Introduction: A Colorful Chemical Encounter
When aqueous solutions of ferric chloride (FeCl₃) and sodium hydroxide (NaOH) are mixed, a striking transformation occurs. The initially clear solutions react to produce a reddish-brown, gelatinous precipitate. This precipitate is ferric hydroxide, Fe(OH)₃, while the remaining solution contains sodium chloride, NaCl, a common table salt. This reaction is a classic example of a double displacement reaction, specifically a precipitation reaction, where an insoluble solid (the precipitate) forms from the reaction of two soluble ionic compounds. The vibrant color change alone makes this reaction a captivating demonstration of chemical principles.
The Balanced Chemical Equation and Net Ionic Equation
The overall balanced chemical equation for the reaction is:
FeCl₃(aq) + 3NaOH(aq) → Fe(OH)₃(s) + 3NaCl(aq)
This equation shows that one mole of ferric chloride reacts with three moles of sodium hydroxide to produce one mole of ferric hydroxide precipitate and three moles of sodium chloride in solution. The (aq) indicates that the substance is dissolved in water (aqueous), while (s) indicates a solid precipitate.
To better understand the underlying ionic interactions, we can write the net ionic equation. This equation focuses only on the species directly involved in the precipitate formation:
Fe³⁺(aq) + 3OH⁻(aq) → Fe(OH)₃(s)
This equation highlights the combination of ferric ions (Fe³⁺) and hydroxide ions (OH⁻) from the respective solutions to form the insoluble ferric hydroxide. The sodium (Na⁺) and chloride (Cl⁻) ions remain in solution as spectator ions, meaning they do not participate directly in the precipitation reaction.
A Detailed Explanation of the Reaction Mechanism
The reaction mechanism is relatively straightforward. Both FeCl₃ and NaOH are strong electrolytes, meaning they completely dissociate into their constituent ions in aqueous solution:
FeCl₃(aq) → Fe³⁺(aq) + 3Cl⁻(aq)
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
When these solutions are mixed, the ferric ions (Fe³⁺) and hydroxide ions (OH⁻) encounter each other. Ferric hydroxide, Fe(OH)₃, has a very low solubility product constant (Ksp), meaning it is significantly less soluble in water than the reactants. As a result, the ferric and hydroxide ions combine to form a solid precipitate, leaving the sodium and chloride ions dissolved in the solution. The formation of the precipitate is driven by the decrease in the Gibbs Free Energy of the system. This means the reaction is thermodynamically favorable.
Solubility and the Solubility Product Constant (Ksp)
The insolubility of ferric hydroxide is key to understanding this precipitation reaction. The solubility product constant (Ksp) is an equilibrium constant that describes the solubility of a sparingly soluble ionic compound. A low Ksp value indicates low solubility. For Fe(OH)₃, the Ksp is extremely small, meaning that only a very small amount of Fe(OH)₃ will dissolve in water at equilibrium. When the concentration of Fe³⁺ and OH⁻ ions exceeds the Ksp value, the precipitation of Fe(OH)₃ occurs to re-establish equilibrium.
Practical Applications of this Reaction
This reaction, seemingly simple, has several important practical applications:
-
Water Treatment: Ferric chloride is used as a coagulant in water treatment plants. The addition of NaOH or other bases causes the precipitation of Fe(OH)₃, which helps to remove impurities and suspended solids from the water. The Fe(OH)₃ precipitate acts as a "clumping agent," binding to smaller particles and facilitating their sedimentation.
-
Wastewater Treatment: Similar to water treatment, Fe(OH)₃ precipitation helps remove heavy metals and other pollutants from wastewater. The ferric hydroxide can adsorb these contaminants, effectively removing them from the water.
-
Chemical Synthesis: Ferric hydroxide can be used as a precursor in the synthesis of other iron compounds. The controlled precipitation allows for the creation of specific iron oxide nanoparticles with different sizes and properties.
-
Analytical Chemistry: The precipitation of Fe(OH)₃ can be utilized in gravimetric analysis to determine the concentration of iron in a sample. By carefully weighing the collected precipitate, the iron content can be calculated.
-
Pigment Production: Iron oxides, produced through reactions involving Fe(OH)₃, are widely used as pigments in various applications.
Safety Precautions and Handling
While the reaction itself is not inherently dangerous, certain safety precautions should be followed when handling the chemicals involved:
-
FeCl₃: Ferric chloride is a corrosive substance. Appropriate eye protection and gloves should be worn when handling it. Skin contact should be avoided.
-
NaOH: Sodium hydroxide is also corrosive and can cause severe skin burns. Similar safety precautions as for FeCl₃ should be employed.
-
Waste Disposal: The ferric hydroxide precipitate and the remaining solution should be disposed of properly according to local regulations. They should not be simply poured down the drain.
Frequently Asked Questions (FAQ)
Q: What color is the Fe(OH)₃ precipitate?
A: The Fe(OH)₃ precipitate is typically a reddish-brown gelatinous solid.
Q: Is the reaction reversible?
A: The reaction is essentially irreversible under normal conditions. The low Ksp of Fe(OH)₃ makes it very unlikely to redissolve spontaneously.
Q: Can other bases be used instead of NaOH?
A: Yes, other strong bases like KOH (potassium hydroxide) will produce a similar precipitation reaction with FeCl₃.
Q: What happens if you add excess NaOH?
A: Adding excess NaOH can lead to the formation of soluble hydroxo complexes of iron, such as [Fe(OH)₄]⁻, changing the appearance of the solution and potentially affecting the efficiency of the precipitation process.
Q: Can this reaction be used to identify the presence of Fe³⁺ ions?
A: Yes, the formation of a reddish-brown precipitate upon addition of a base is a qualitative test for the presence of Fe³⁺ ions in a solution.
Conclusion: A Foundation of Chemical Understanding
The reaction between ferric chloride and sodium hydroxide is a visually striking and conceptually significant example of a precipitation reaction. Understanding this reaction provides a solid foundation for grasping fundamental concepts in chemistry, including solubility, stoichiometry, and ionic bonding. Its practical applications across various fields highlight the importance of seemingly simple chemical processes in our everyday lives and industrial processes. This reaction, while simple in its execution, offers a rich learning experience for anyone exploring the wonders of chemical transformations. The careful observation and analysis of this reaction can lead to a deeper appreciation of the principles governing the behavior of matter at a molecular level.
Latest Posts
Latest Posts
-
Molar Mass Of K3 Fe C2o4 3 3h2o
Sep 22, 2025
-
Parallelogram Abcd With Diagonals Ac And Bd
Sep 22, 2025
-
A Walkway Is 4 Feet Wide
Sep 22, 2025
-
What Is The Oxygen To Sulfur Mass Ratio Of Sulfur Dioxide
Sep 22, 2025
-
Click On The Measure Of The Angle
Sep 22, 2025
Related Post
Thank you for visiting our website which covers about Fecl3 Naoh Fe Oh 3 Nacl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.