Draw A Lewis Diagram For Ch3ch2oh.
faraar
Sep 12, 2025 · 6 min read
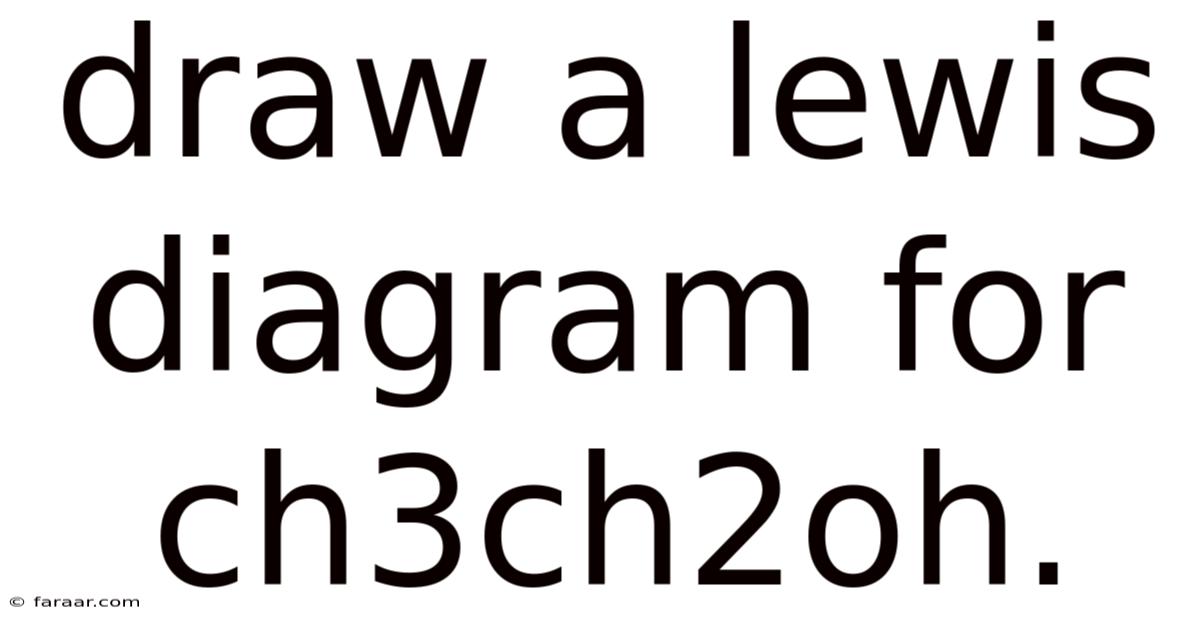
Table of Contents
Drawing the Lewis Diagram for CH₃CH₂OH (Ethanol)
Understanding how to draw Lewis diagrams, also known as Lewis structures, is fundamental to grasping the basics of chemistry. These diagrams visually represent the valence electrons of atoms within a molecule, showing how atoms bond and the distribution of electrons. This article will guide you through the step-by-step process of drawing the Lewis diagram for CH₃CH₂OH, commonly known as ethanol, a simple organic alcohol, and explore the underlying principles involved. This guide will cover not only the mechanics of drawing the diagram but also delve into the scientific reasoning behind the structure, addressing common questions and misconceptions.
Introduction: Understanding Lewis Diagrams and Valence Electrons
A Lewis diagram is a simplified representation of a molecule's structure, showcasing the arrangement of atoms and the bonding electrons. The core concept relies on the valence electrons, the electrons in the outermost shell of an atom that participate in chemical bonding. These valence electrons are crucial in determining the reactivity and bonding behavior of an element.
To successfully draw a Lewis diagram, you need to know the number of valence electrons each atom possesses. This is determined by the atom's position in the periodic table. For the atoms in CH₃CH₂OH (ethanol):
- Carbon (C): Group 14, 4 valence electrons
- Hydrogen (H): Group 1, 1 valence electron
- Oxygen (O): Group 16, 6 valence electrons
Step-by-Step Guide to Drawing the Lewis Diagram for CH₃CH₂OH
-
Count the Total Valence Electrons:
First, let's tally the total number of valence electrons in the ethanol molecule:
- 2 Carbon atoms × 4 valence electrons/atom = 8 electrons
- 6 Hydrogen atoms × 1 valence electron/atom = 6 electrons
- 1 Oxygen atom × 6 valence electrons/atom = 6 electrons
- Total: 20 valence electrons
-
Identify the Central Atom(s):
In ethanol, the carbon atoms form the backbone of the molecule. They will be the central atoms. Oxygen is also a crucial central atom, connecting to one of the carbon atoms.
-
Arrange the Atoms:
Connect the atoms with single bonds, forming a skeletal structure. Remember, Carbon tends to form four bonds, Oxygen two, and Hydrogen one. A typical arrangement for ethanol is a chain structure: C-C-O-H, with the remaining Hydrogens bonded to the Carbons. This arrangement represents the connectivity of the atoms.
-
Distribute the Remaining Electrons:
Subtract the number of electrons used in the single bonds (2 electrons per bond) from the total number of valence electrons.
- Total valence electrons: 20
- Electrons used in single bonds (7 bonds × 2 electrons/bond): 14 electrons
- Remaining electrons: 20 - 14 = 6 electrons
Now, distribute these remaining 6 electrons around the atoms to satisfy the octet rule (except for Hydrogen, which follows the duet rule). The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons. Hydrogen only needs two electrons for stability (duet rule).
Start by placing lone pairs (pairs of electrons) on the Oxygen atom. Oxygen needs two more electron pairs to complete its octet.
-
Check the Octet Rule:
Examine the Lewis diagram to ensure all atoms (except Hydrogen) have eight valence electrons surrounding them. If any atom does not have a complete octet, you may need to form double or triple bonds by moving lone pairs to form additional bonds. In this case, all atoms have satisfied the octet or duet rule.
-
Formal Charges:
While not always necessary for simple molecules, calculating formal charges can be helpful. The formal charge of an atom is the difference between the number of valence electrons in the free atom and the number of electrons assigned to the atom in the Lewis structure. The formula is:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - 1/2(Bonding Electrons)
For ethanol, all atoms should have a formal charge of zero, indicating a stable structure.
The Completed Lewis Diagram for CH₃CH₂OH
The final Lewis diagram will look like this:
H H H
| | |
H - C - C - O - H
| |
H H
Each line represents a single covalent bond (2 electrons shared). The Oxygen atom will have two lone pairs of electrons. All atoms satisfy the octet (or duet) rule.
Explanation of Bonding and Molecular Geometry
The Lewis diagram reveals the bonding within ethanol. The C-C bond is a single covalent bond, sharing two electrons between the two carbon atoms. The C-H bonds are also single covalent bonds. The C-O bond is a single covalent bond, and the O-H bond represents the hydroxyl group (-OH), characterizing alcohols.
The molecular geometry around each carbon atom is tetrahedral, with bond angles of approximately 109.5 degrees. The geometry around the oxygen atom is bent due to the presence of two lone pairs of electrons.
FAQ: Common Questions and Misconceptions
-
Q: Why is the Oxygen atom crucial?
A: The Oxygen atom is vital because it introduces polarity into the molecule. The O-H bond is highly polar due to the significant electronegativity difference between Oxygen and Hydrogen. This polarity influences the physical and chemical properties of ethanol, such as its solubility in water and its ability to participate in hydrogen bonding.
-
Q: Can I draw the ethanol structure differently?
A: While the chain structure shown above is the most common and accurate representation, other minor variations in the arrangement of atoms on the page are possible, as long as the connectivity remains the same and the octet/duet rules are followed.
-
Q: What about resonance structures?
A: Ethanol does not exhibit resonance structures. Resonance occurs when multiple valid Lewis structures can be drawn for a molecule, and the actual structure is a hybrid of these contributors. Ethanol’s structure is straightforward, with a single, most stable configuration.
-
Q: How does the Lewis diagram relate to the 3D structure?
A: The Lewis diagram provides a 2D representation of the molecule. While it shows the connectivity and electron distribution, it doesn't accurately portray the three-dimensional shape. More advanced methods, such as VSEPR (Valence Shell Electron Pair Repulsion) theory, are needed to predict the actual 3D shape of the molecule.
Conclusion: Mastering Lewis Diagrams for Deeper Understanding
Drawing Lewis diagrams is a critical skill in chemistry. It provides a foundational understanding of molecular structure, bonding, and electron distribution. By following the systematic steps outlined above and understanding the underlying principles, you can successfully draw the Lewis diagram for ethanol (CH₃CH₂OH) and other molecules. Remember that mastering this skill will significantly enhance your comprehension of chemical concepts and pave the way for tackling more complex chemical structures and reactions. The ability to visualize molecular structures is key to unlocking a deeper appreciation of the world around us at a molecular level.
Latest Posts
Latest Posts
-
What Is The Value Of X 45
Sep 12, 2025
-
What Is Less Than 1 2 Inch
Sep 12, 2025
-
How To Find Upper And Lower Limits In Statistics
Sep 12, 2025
-
Express Each Relation As A Table Graph And Mapping
Sep 12, 2025
-
1 2 3 Cup Equals How Many Ounces
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Draw A Lewis Diagram For Ch3ch2oh. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.