Ch3 Ch2 4ch3 O2 Co2 H2o
faraar
Sep 12, 2025 · 6 min read
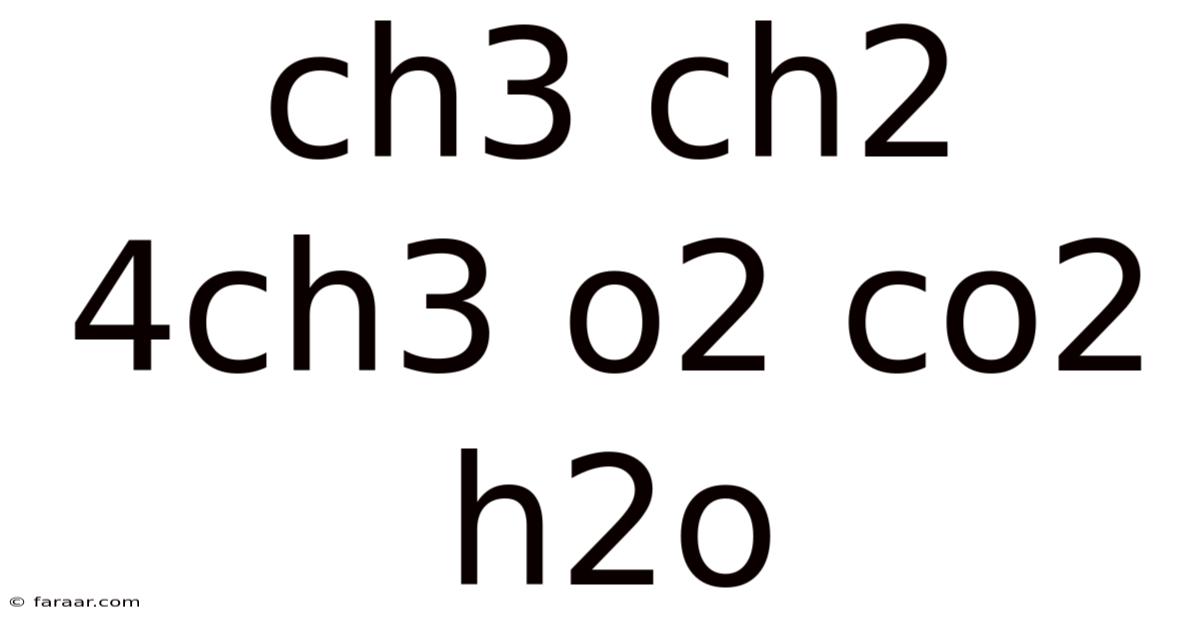
Table of Contents
The Combustion of Alkanes: A Deep Dive into CH3CH2CH2CH3 + O2 → CO2 + H2O
This article explores the complete combustion of butane (CH3CH2CH2CH3), a common alkane, with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). We'll delve into the balanced chemical equation, the step-by-step process, the underlying chemistry, and answer frequently asked questions. Understanding this reaction is crucial for comprehending fundamental concepts in organic chemistry and combustion processes.
Introduction: Understanding Combustion
Combustion, simply put, is a rapid chemical reaction between a substance and an oxidant, usually oxygen, that produces heat and light. In the case of alkanes like butane, this reaction involves the breaking of carbon-hydrogen and carbon-carbon bonds, followed by the formation of new bonds to create carbon dioxide and water. The reaction releases a significant amount of energy, making alkanes valuable fuels. This article will focus on the complete combustion of butane, meaning sufficient oxygen is present for the complete oxidation of all carbon atoms to carbon dioxide and all hydrogen atoms to water. Incomplete combustion, where there's insufficient oxygen, results in the formation of carbon monoxide (CO) and soot (carbon particles), which are highly toxic and environmentally damaging.
The Balanced Chemical Equation: The Foundation of the Reaction
The complete combustion of butane can be represented by the following balanced chemical equation:
2CH₃CH₂CH₂CH₃ + 13O₂ → 8CO₂ + 10H₂O
This equation tells us that two molecules of butane react with thirteen molecules of oxygen to produce eight molecules of carbon dioxide and ten molecules of water. The balancing of this equation is critical; it ensures that the number of atoms of each element is the same on both sides of the equation, obeying the law of conservation of mass. Notice that the equation reflects the complete oxidation of butane; all carbon atoms end up in CO2, and all hydrogen atoms end up in H2O.
Step-by-Step Breakdown of the Combustion Process
While the balanced equation provides a concise summary, the actual combustion process occurs through a series of complex steps involving free radicals. These steps are generally too intricate to detail fully here, but we can outline the key stages:
-
Initiation: The reaction begins with the initiation step, where the high temperature (typically from a spark or flame) breaks down oxygen molecules (O2) into highly reactive oxygen radicals (•O). These radicals are highly unstable and readily react with other molecules.
-
Propagation: Oxygen radicals then attack butane molecules, abstracting hydrogen atoms and forming butane radicals (•CH₂CH₂CH₂CH₃). This step generates more radicals, creating a chain reaction. Further reactions involve the breaking of carbon-carbon bonds and the formation of smaller alkyl radicals.
-
Oxidation: The alkyl radicals react with oxygen to form peroxy radicals (ROO•). These radicals undergo further reactions, ultimately leading to the formation of carbon dioxide and water.
-
Termination: The chain reaction eventually terminates when two radicals combine, forming stable molecules, thereby stopping the radical propagation.
This detailed mechanism involves numerous intermediate species and reactions, making it a complex process to fully illustrate within the scope of this article. However, the overall result, as represented by the balanced equation, remains consistent.
The Chemistry Behind the Combustion: Bond Energies and Energy Release
The combustion of butane is highly exothermic, meaning it releases a significant amount of energy. This energy release is a consequence of the difference in bond energies between the reactants (butane and oxygen) and the products (carbon dioxide and water).
-
Reactants: Butane contains strong C-C and C-H bonds. Oxygen molecules (O=O) also have a relatively strong double bond.
-
Products: Carbon dioxide contains strong C=O double bonds, and water contains strong O-H bonds.
The energy released during combustion comes from the difference between the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products. Since the bonds in the products are stronger overall, the net energy change is negative – energy is released as heat and light. This energy difference is known as the enthalpy change (ΔH) of the reaction, and it's a significant factor in the usefulness of butane as a fuel.
Environmental Considerations: CO2 and its Impact
The complete combustion of butane produces carbon dioxide (CO2), a major greenhouse gas. The increase in atmospheric CO2 concentration due to the burning of fossil fuels, including butane, is a primary contributor to global warming and climate change. While butane is a relatively clean-burning fuel compared to some others (producing less particulate matter), the CO2 produced still has a significant environmental impact. Efforts to mitigate this impact include exploring alternative renewable energy sources and developing technologies for carbon capture and storage.
Frequently Asked Questions (FAQ)
Q1: What happens if there isn't enough oxygen for complete combustion?
A1: Incomplete combustion occurs, resulting in the formation of carbon monoxide (CO) and potentially soot (carbon particles). Carbon monoxide is a highly toxic gas that can be fatal if inhaled. Soot contributes to air pollution and has detrimental health effects.
Q2: Is butane a cleaner-burning fuel than other hydrocarbons?
A2: Compared to some heavier hydrocarbons, butane is considered relatively clean-burning because it produces less soot and particulate matter during complete combustion. However, it still produces CO2, a greenhouse gas.
Q3: What are the applications of butane combustion?
A3: Butane combustion finds numerous applications, including powering lighters, camping stoves, and some types of gas grills. It's also used as a propellant in aerosols and as a refrigerant.
Q4: Can the combustion of butane be controlled?
A4: Yes, the combustion of butane can be precisely controlled by adjusting the fuel-to-air ratio and the ignition source. This control is essential for its safe and efficient use in various applications.
Q5: What are the safety precautions when working with butane?
A5: Butane is highly flammable and should be handled with care. It should be stored in well-ventilated areas away from ignition sources. Always follow the manufacturer's safety instructions when using butane-powered devices.
Conclusion: Understanding the Fundamentals of Combustion
The complete combustion of butane, represented by the equation 2CH₃CH₂CH₂CH₃ + 13O₂ → 8CO₂ + 10H₂O, is a fundamental chemical reaction with significant implications for energy production and environmental science. Understanding the balanced equation, the step-by-step process, the underlying chemistry of bond energies, and the environmental consequences of CO2 emissions is crucial for a comprehensive grasp of this important reaction. While the detailed reaction mechanism is complex, the overall outcome – the transformation of a hydrocarbon fuel into carbon dioxide and water – remains a cornerstone of our understanding of combustion processes. Further research and development in energy technologies are crucial to mitigate the negative environmental impacts associated with the combustion of fossil fuels like butane.
Latest Posts
Latest Posts
-
3 Inches By 3 Inches Square
Sep 12, 2025
-
Are Fungi Cells Prokaryotic Or Eukaryotic
Sep 12, 2025
-
How To Calculate Percent Recovery Recrystallization
Sep 12, 2025
-
Give A Geometric Description Of The Following Set Of Points
Sep 12, 2025
-
2 3 Cup Is How Many 1 3 Cups
Sep 12, 2025
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 4ch3 O2 Co2 H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.