Bacl2 + Na2co3 Net Ionic Equation
faraar
Sep 20, 2025 · 6 min read
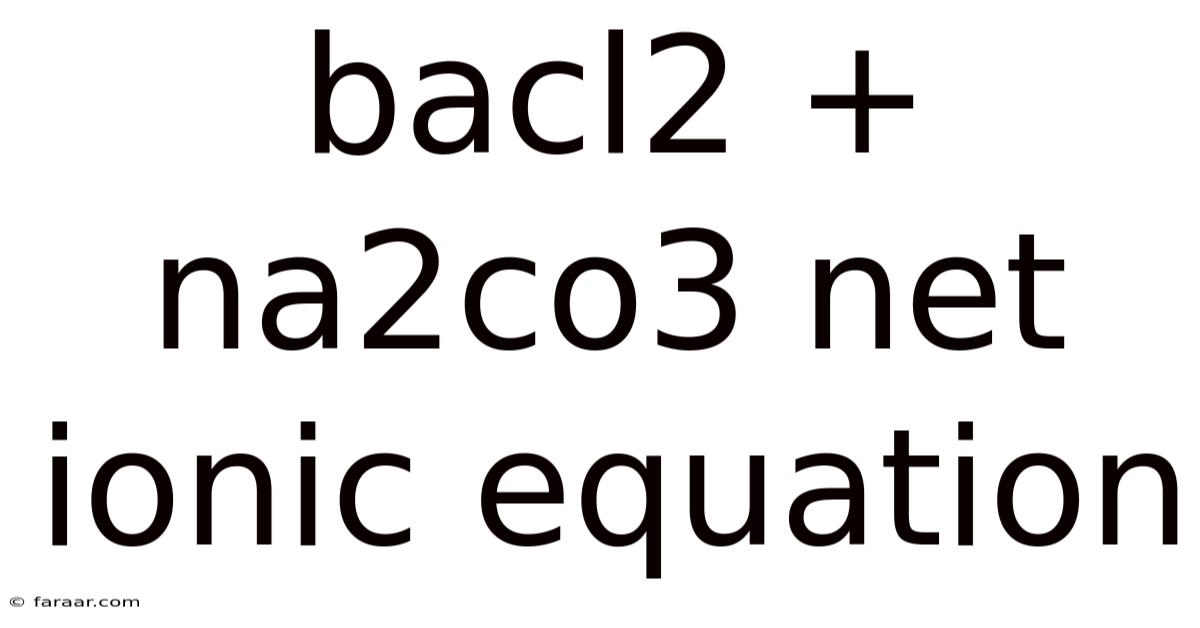
Table of Contents
Understanding the Net Ionic Equation: BaCl₂ + Na₂CO₃
The reaction between barium chloride (BaCl₂) and sodium carbonate (Na₂CO₃) is a classic example used in chemistry to illustrate the concept of net ionic equations. This seemingly simple reaction offers a rich learning opportunity to understand precipitation reactions, spectator ions, and the importance of writing balanced chemical equations. This article will delve into the details of this reaction, explaining the process of deriving the net ionic equation and exploring the underlying chemistry. We will cover the complete reaction mechanism, provide step-by-step instructions, and address frequently asked questions.
Introduction: Precipitation Reactions and Ionic Compounds
Before diving into the specifics of BaCl₂ and Na₂CO₃, let's establish a foundational understanding. This reaction is a precipitation reaction, a type of double displacement reaction where two soluble ionic compounds react in a solution to form an insoluble solid called a precipitate. The solubility of ionic compounds is crucial; it dictates whether a precipitate will form. Solubility rules, which outline the solubility of various ionic compounds in water, are essential tools for predicting reaction outcomes.
Both barium chloride (BaCl₂) and sodium carbonate (Na₂CO₃) are highly soluble ionic compounds. When dissolved in water, they dissociate into their constituent ions:
- BaCl₂(aq) → Ba²⁺(aq) + 2Cl⁻(aq)
- Na₂CO₃(aq) → 2Na⁺(aq) + CO₃²⁻(aq)
The "(aq)" indicates that these species are aqueous – dissolved in water. The reaction between these two compounds leads to the formation of barium carbonate (BaCO₃), which is insoluble in water, and sodium chloride (NaCl), which remains soluble.
Steps to Derive the Net Ionic Equation for BaCl₂ + Na₂CO₃
Deriving the net ionic equation involves a series of steps:
-
Write the balanced molecular equation: This equation shows the complete chemical formulas of all reactants and products.
BaCl₂(aq) + Na₂CO₃(aq) → BaCO₃(s) + 2NaCl(aq)
Note that this equation is balanced in terms of both atoms and charges.
-
Write the complete ionic equation: This equation shows all the ions present in the solution, both reactants and products, in their dissociated form. Remember that soluble ionic compounds dissociate completely in solution. Insoluble compounds remain as molecules.
Ba²⁺(aq) + 2Cl⁻(aq) + 2Na⁺(aq) + CO₃²⁻(aq) → BaCO₃(s) + 2Na⁺(aq) + 2Cl⁻(aq)
-
Identify and cancel spectator ions: Spectator ions are ions that appear on both sides of the complete ionic equation. They do not participate directly in the reaction. In this case, Na⁺(aq) and Cl⁻(aq) are spectator ions. Cancel them out from both sides of the equation.
-
Write the net ionic equation: This equation shows only the ions that directly participate in the reaction.
Ba²⁺(aq) + CO₃²⁻(aq) → BaCO₃(s)
This is the net ionic equation for the reaction between barium chloride and sodium carbonate. It clearly shows the formation of the solid barium carbonate precipitate from its constituent ions.
Detailed Explanation of the Reaction Mechanism
The reaction proceeds through the electrostatic attraction between the barium ions (Ba²⁺) and the carbonate ions (CO₃²⁻). These ions have opposite charges, leading to a strong electrostatic force that overcomes the attractive forces between the ions and the water molecules. The result is the formation of a solid barium carbonate precipitate. The precipitate is a crystalline solid that settles out of the solution.
The solubility of barium carbonate is extremely low, meaning the equilibrium strongly favors the solid state. This is why the reaction proceeds almost completely to the formation of the precipitate. The sodium and chloride ions, being spectator ions, remain dissolved in the solution.
Factors Affecting the Reaction
Several factors can affect the rate and extent of the reaction:
-
Concentration of reactants: Higher concentrations of BaCl₂ and Na₂CO₃ will lead to a faster reaction rate and potentially a more complete precipitation.
-
Temperature: Increasing the temperature can increase the reaction rate, although the solubility of BaCO₃ might also slightly increase, reducing the amount of precipitate formed.
-
Presence of other ions: The presence of other ions in the solution can influence the ionic strength, affecting the solubility of BaCO₃ and the overall reaction rate.
Applications of the BaCl₂ + Na₂CO₃ Reaction
This seemingly simple reaction has several practical applications:
-
Qualitative analysis: The formation of a white precipitate of barium carbonate can be used as a qualitative test for the presence of either barium ions (Ba²⁺) or carbonate ions (CO₃²⁻) in a solution.
-
Preparation of barium carbonate: While not the most efficient method, this reaction can be used to prepare small quantities of pure barium carbonate in a laboratory setting.
-
Understanding precipitation reactions: This reaction serves as an excellent example to teach the fundamental principles of precipitation reactions, net ionic equations, and spectator ions in introductory chemistry courses.
Frequently Asked Questions (FAQ)
Q1: Why is the net ionic equation important?
A1: The net ionic equation simplifies the reaction by focusing on the essential species involved in the formation of the precipitate. It eliminates the spectator ions, which are not directly involved in the chemical change. It helps us understand the core chemical process occurring without unnecessary details.
Q2: What are the safety precautions when performing this reaction?
A2: Barium compounds are toxic. Appropriate safety measures should always be taken, including wearing safety goggles and gloves. The reaction should be performed under adequate ventilation. Proper disposal of the waste products is crucial.
Q3: Can the reaction be reversed?
A3: While the precipitation of BaCO₃ is essentially irreversible under normal conditions, it is possible to dissolve the precipitate under specific conditions, such as by reacting it with a strong acid. This would require a different chemical reaction.
Q4: What would happen if we used a different soluble barium salt instead of BaCl₂?
A4: The net ionic equation would remain the same. The anion of the barium salt would simply become a spectator ion. For example, if we used barium nitrate (Ba(NO₃)₂), the nitrate ion (NO₃⁻) would become the spectator ion.
Q5: How can I visually observe the reaction?
A5: When you mix solutions of BaCl₂ and Na₂CO₃, you will observe the immediate formation of a cloudy white precipitate of barium carbonate. The solution will become turbid, and over time, the precipitate will settle at the bottom of the container.
Conclusion
The reaction between barium chloride and sodium carbonate is a fundamental example illustrating the concept of precipitation reactions and the importance of net ionic equations. By understanding the step-by-step process of deriving the net ionic equation, we can gain a deeper appreciation for the underlying chemistry and its various applications. This reaction provides a valuable learning opportunity to strengthen our understanding of chemical reactions and the behavior of ions in solution. The principles discussed here are applicable to a wide range of chemical reactions and are essential for a comprehensive grasp of inorganic chemistry. Remember to always prioritize safety when conducting any chemical experiments.
Latest Posts
Latest Posts
-
What Is The Value Of The Y Coordinate Of Point A
Sep 20, 2025
-
Can Compounds Be Broken Down Into Simpler Substances
Sep 20, 2025
-
Private Softball Pitching Lessons Near Me
Sep 20, 2025
-
How Do You Find The Base Of A Trapezoid
Sep 20, 2025
-
Is Calcium Chloride A Strong Electrolyte
Sep 20, 2025
Related Post
Thank you for visiting our website which covers about Bacl2 + Na2co3 Net Ionic Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.