Atomic Mass Is Equal To The Number Of
faraar
Sep 21, 2025 · 6 min read
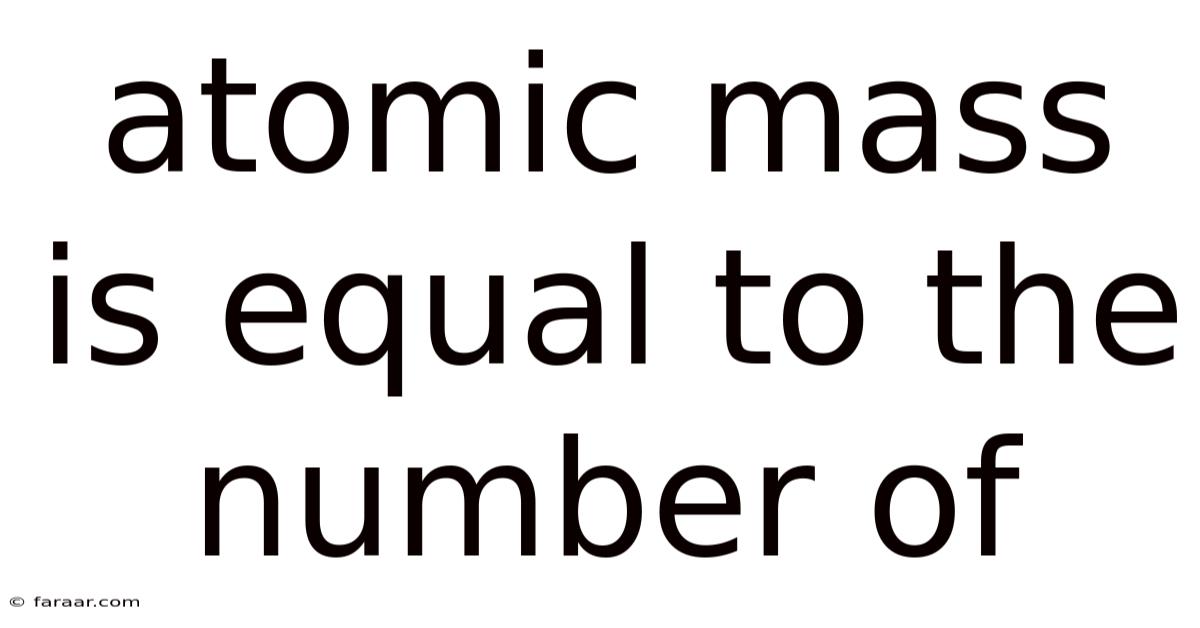
Table of Contents
Atomic Mass: More Than Just the Number of Protons
Understanding atomic mass is fundamental to grasping the nature of matter and the behavior of elements. While often simplified as "the number of protons," this statement is only partially true. This comprehensive article will delve into the intricacies of atomic mass, explaining what it truly represents, how it's determined, and its significance in various scientific fields. We'll explore the contributions of protons, neutrons, and isotopes, unraveling the nuances behind this crucial atomic property.
Introduction: Unveiling the Atomic Nucleus
Every atom, the fundamental building block of matter, consists of a nucleus surrounded by orbiting electrons. The nucleus, incredibly dense and positively charged, houses two types of particles: protons and neutrons. These subatomic particles determine the atom's identity and its behavior in chemical reactions. While the number of protons defines the atomic number and thus the element itself, the atomic mass reflects the total mass of the atom, encompassing both protons and neutrons. The misconception that atomic mass equals only the number of protons stems from a simplified view ignoring the significant contribution of neutrons.
Protons: Defining the Element
Protons carry a positive charge (+1) and possess a mass approximately equal to 1 atomic mass unit (amu). The number of protons in an atom's nucleus is its atomic number, a unique identifier for each element on the periodic table. Hydrogen (H), with one proton, has an atomic number of 1; helium (He), with two protons, has an atomic number of 2, and so on. The atomic number dictates the element's chemical properties, determining how it interacts with other elements to form compounds.
Neutrons: Adding Mass Without Changing Identity
Neutrons, as their name suggests, are electrically neutral particles residing in the atomic nucleus. Their mass is also approximately 1 amu, very close to that of a proton. Unlike protons, the number of neutrons in an atom's nucleus can vary without changing the element's identity. This variability leads to the existence of isotopes.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element (same atomic number) but with different numbers of neutrons. This means they have the same number of protons but different mass numbers. The mass number is the sum of the protons and neutrons in the nucleus. For example, carbon (C) has an atomic number of 6. The most common isotope, carbon-12 (¹²C), has 6 protons and 6 neutrons (mass number = 12). However, carbon-13 (¹³C) has 6 protons and 7 neutrons (mass number = 13), and carbon-14 (¹⁴C) has 6 protons and 8 neutrons (mass number = 14). All three are isotopes of carbon, exhibiting slightly different physical properties due to the varied neutron numbers, but sharing the same chemical behavior determined by their six protons.
Atomic Mass Unit (amu): A Standard of Measurement
The atomic mass unit (amu), also known as the dalton (Da), is a standard unit for expressing the mass of atoms and molecules. It's defined as one-twelfth the mass of a single carbon-12 atom. This means that one amu is approximately 1.66 x 10⁻²⁴ grams. Using amu allows scientists to conveniently compare the masses of different atoms and molecules.
Calculating Atomic Mass: Averaging Isotopic Abundances
The atomic mass listed on the periodic table for an element isn't the mass number of a single isotope; it's a weighted average of the masses of all naturally occurring isotopes of that element. The weighting considers the relative abundance of each isotope in nature. For example, chlorine (Cl) has two major isotopes: chlorine-35 (⁷⁵% abundance) and chlorine-37 (²⁵% abundance). The atomic mass of chlorine is calculated as follows:
(0.75 x 35 amu) + (0.25 x 37 amu) ≈ 35.5 amu
This weighted average reflects the typical mass of a chlorine atom found in nature.
The Significance of Atomic Mass in Various Scientific Fields
Understanding atomic mass is crucial across multiple scientific disciplines:
-
Chemistry: Atomic mass is essential for stoichiometric calculations, determining the amount of reactants and products in chemical reactions. It's fundamental to understanding molar mass and mole concepts, critical for quantitative analysis in chemistry.
-
Nuclear Physics: In nuclear physics, atomic mass is crucial for understanding nuclear reactions, including fission and fusion. The mass defect (the difference between the mass of the nucleus and the sum of the masses of its individual protons and neutrons) is directly related to the binding energy holding the nucleus together.
-
Mass Spectrometry: This analytical technique utilizes the mass-to-charge ratio of ions to identify and quantify the different isotopes present in a sample. Atomic mass is central to interpreting the resulting mass spectra.
-
Geochemistry and Cosmochemistry: Isotopic ratios of elements are powerful tools for dating geological samples and understanding planetary formation processes. The relative abundances of isotopes, linked to their atomic masses, provide insights into the history of Earth and the solar system.
Frequently Asked Questions (FAQ)
-
Q: Is atomic mass always a whole number?
- A: No, atomic mass is usually a decimal number because it represents a weighted average of the masses of different isotopes, each with a different mass number (a whole number).
-
Q: What is the difference between atomic mass and mass number?
- A: Atomic mass is the weighted average mass of all isotopes of an element, while the mass number refers to the total number of protons and neutrons in a specific isotope's nucleus.
-
Q: How accurate are the atomic mass values on the periodic table?
- A: The values are highly accurate, reflecting extensive experimental measurements and refinements over time. Small variations may exist due to ongoing research and improvements in measurement techniques.
-
Q: Can atomic mass change?
- A: The atomic mass of an element as listed on the periodic table represents a constant weighted average based on the naturally occurring isotopic abundances. However, the isotopic composition of a sample can vary slightly depending on its origin. In nuclear reactions, the atomic mass of the nuclei involved changes directly.
Conclusion: A Deeper Understanding of Atomic Structure
Atomic mass, while often simplified, is a far more nuanced concept than merely the number of protons. It's a weighted average reflecting the contributions of both protons and neutrons, accounting for the natural isotopic variations of each element. This understanding is pivotal for comprehending the fundamental properties of matter, its behavior in chemical and nuclear reactions, and its applications across various scientific fields. Appreciating the interplay between protons, neutrons, and isotopes leads to a more profound comprehension of atomic structure and its profound implications in our understanding of the universe. The seemingly simple number representing atomic mass embodies a wealth of scientific knowledge and opens doors to exploration across countless scientific disciplines.
Latest Posts
Latest Posts
-
Two Angles Form A Linear Pair
Sep 21, 2025
-
How Many Hundreds In 10 000
Sep 21, 2025
-
Is Potassium Hydroxide A Strong Electrolyte
Sep 21, 2025
-
Does Oxygen Lose Or Gain Electrons
Sep 21, 2025
-
How Much Is 2 3 Of A Pound
Sep 21, 2025
Related Post
Thank you for visiting our website which covers about Atomic Mass Is Equal To The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.