Atomic Mass Equals The Number Of
faraar
Aug 28, 2025 · 6 min read
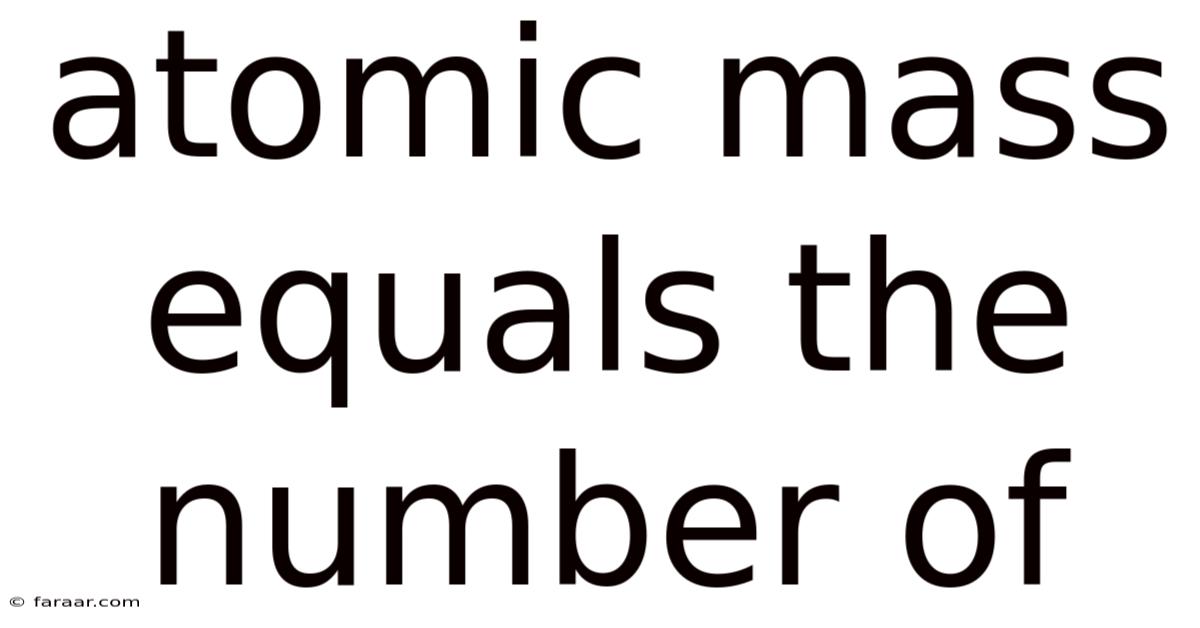
Table of Contents
Atomic Mass: Unveiling the Secrets Within the Atom
Atomic mass, also known as atomic weight, is a fundamental concept in chemistry and physics. Understanding what atomic mass equals is crucial to grasping the behavior of atoms, molecules, and the very fabric of matter. This comprehensive guide will explore the intricacies of atomic mass, explaining what it represents, how it's calculated, and its significance in various scientific fields. We'll delve into isotopes, their contribution to atomic mass, and address common misconceptions.
What Does Atomic Mass Represent?
Simply put, atomic mass represents the average mass of an atom of an element, relative to the mass of a carbon-12 atom, which is defined as exactly 12 atomic mass units (amu). It's not simply the sum of protons and neutrons, as we'll see later. This average takes into account the different isotopes of an element, weighted by their relative abundance in nature.
Imagine you have a bag of marbles. Some are red, some are blue. Each color represents a different isotope of an element. The atomic mass is like calculating the average weight of a single marble, considering both the weight of each color marble and how many of each color are in the bag.
The Building Blocks: Protons, Neutrons, and Electrons
Before diving deeper into atomic mass calculation, it's essential to understand the subatomic particles that constitute an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's atomic number and its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus. Their mass is negligible compared to protons and neutrons and doesn't significantly contribute to the atomic mass.
The mass of a single proton is approximately 1 amu, and the mass of a single neutron is also approximately 1 amu. The mass of an electron is significantly smaller, about 1/1836 amu. This is why the electron's mass is often ignored when calculating atomic mass.
Isotopes: The Key to Understanding Atomic Mass
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number but different mass numbers (the sum of protons and neutrons). For example, carbon-12 (¹²C) has 6 protons and 6 neutrons, while carbon-13 (¹³C) has 6 protons and 7 neutrons. Both are isotopes of carbon, but they have different masses.
The existence of isotopes is crucial because the atomic mass listed on the periodic table isn't the mass of a single isotope, but rather a weighted average of all the naturally occurring isotopes of that element. This weighted average accounts for the relative abundance of each isotope.
Calculating Atomic Mass: A Weighted Average
To calculate the atomic mass, we use a weighted average formula:
Atomic Mass = (mass of isotope 1 × % abundance of isotope 1) + (mass of isotope 2 × % abundance of isotope 2) + ...
Let's consider chlorine as an example. Chlorine has two main isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). Chlorine-35 has a mass of approximately 34.97 amu and a natural abundance of about 75.77%. Chlorine-37 has a mass of approximately 36.97 amu and a natural abundance of about 24.23%.
Using the formula:
Atomic Mass of Chlorine ≈ (34.97 amu × 0.7577) + (36.97 amu × 0.2423) ≈ 35.45 amu
This calculated atomic mass of approximately 35.45 amu is what you would find on the periodic table for chlorine. Note that this is an approximation; more precise measurements might yield a slightly different value.
The Significance of Atomic Mass
Atomic mass is a cornerstone of many chemical and physical calculations. Its significance extends to several key areas:
- Stoichiometry: Atomic mass is crucial in stoichiometric calculations, allowing us to determine the quantities of reactants and products in chemical reactions. It enables the conversion between grams and moles, a fundamental unit in chemistry.
- Nuclear Chemistry: Understanding isotopic masses is vital in nuclear chemistry, where the study of nuclear reactions and radioactive decay relies heavily on isotopic masses and their changes during these processes.
- Mass Spectrometry: Mass spectrometry is an analytical technique that uses the mass-to-charge ratio of ions to identify and quantify different molecules and isotopes. Atomic mass is a key parameter in interpreting mass spectrometry data.
- Material Science: Atomic mass influences the properties of materials. For instance, the different isotopes of an element can affect the density and other physical properties of a material.
Beyond the Basics: Advanced Concepts
While the weighted average calculation provides a good understanding of atomic mass, more advanced concepts refine our understanding:
- Mass Defect: The mass of an atom is slightly less than the sum of the masses of its individual protons, neutrons, and electrons. This difference is called the mass defect and is a consequence of Einstein's famous equation, E=mc², representing the energy binding the nucleus together.
- Nuclear Binding Energy: The mass defect is directly related to the nuclear binding energy, the energy required to break apart an atom's nucleus into its constituent protons and neutrons. This energy is a measure of the stability of the nucleus.
- Relative Atomic Mass vs. Standard Atomic Weight: While often used interchangeably, there’s a subtle difference. Relative atomic mass refers to the mass of a single atom relative to ¹²C, while standard atomic weight is the average mass considering the isotopic composition of the element, as it naturally occurs on Earth. This isotopic composition can vary slightly depending on the source of the sample.
Frequently Asked Questions (FAQ)
Q: Why is the atomic mass not a whole number?
A: Because it's a weighted average of the masses of all the naturally occurring isotopes of an element, taking into account their relative abundances. The masses of individual isotopes are close to whole numbers (due to the approximate mass of protons and neutrons), but the weighted average is rarely a whole number.
Q: What is the difference between atomic number and atomic mass?
A: The atomic number represents the number of protons in an atom's nucleus and determines the element's identity. Atomic mass represents the average mass of an atom of that element, considering all its naturally occurring isotopes and their relative abundances.
Q: Can the atomic mass of an element change?
A: The atomic mass reported on the periodic table is a weighted average, and it might change slightly based on updated measurements of isotopic abundances. In specific situations, the isotopic composition can change artificially (e.g., enriched uranium used in nuclear reactors), leading to a different atomic mass for that specific sample.
Q: How are atomic masses measured?
A: Precise measurements of atomic masses are primarily obtained using mass spectrometry. This technique allows for the accurate determination of the mass-to-charge ratio of ions, which is crucial in calculating atomic masses.
Conclusion
Atomic mass is a fundamental concept in chemistry and physics, representing the average mass of an atom of an element, weighted by the natural abundance of its isotopes. Understanding atomic mass is essential for various applications, from stoichiometric calculations to nuclear chemistry and material science. While seemingly a simple concept, delving into isotopes, mass defect, and nuclear binding energy reveals a deeper understanding of the atom's structure and behavior, underscoring its importance in our comprehension of the universe. By understanding atomic mass, we gain a clearer insight into the building blocks of matter and their interactions, shaping our knowledge of the natural world.
Latest Posts
Latest Posts
-
What Was The Official Language Of The Roman Empire
Aug 28, 2025
-
Graph The Line 3x Y 3
Aug 28, 2025
-
Find Radian Measure Of Central Angle
Aug 28, 2025
-
9 Tsp Equals How Many Tbsp
Aug 28, 2025
-
How To Foil With 3 Terms
Aug 28, 2025
Related Post
Thank you for visiting our website which covers about Atomic Mass Equals The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.